info@universallab.org
WhatsApp: +41762172997


Price may vary based on selected options
Delivery time: 1 ~ 2 weeks

AAS (Atomic Absorption Spectroscopy) is a widely used analytical technique for the quantitative determination of metal elements in a variety of sample types. It offers precise and sensitive measurements of elemental concentrations, especially for trace and ultra-trace metals. The basic principle of AAS involves atomizing the sample—typically in a flame or graphite furnace—and measuring the absorption of light at specific wavelengths by free atoms of the target element. Each element absorbs light at a characteristic wavelength, and the amount of light absorbed is directly proportional to the concentration of that element in the sample. Typical applications include the determination of heavy metals such as lead (Pb), cadmium (Cd), arsenic (As), mercury (Hg), and chromium (Cr) in water, soil, food, and biological samples. AAS is also commonly used for measuring calcium (Ca), magnesium (Mg), iron (Fe), and zinc (Zn) in pharmaceutical and nutritional products.
Industries that widely apply AAS include environmental monitoring, food safety, pharmaceuticals, clinical diagnostics, metallurgy, mining, and chemical manufacturing. Key Feature
FAAS (Flame Atomic Absorption Spectroscopy) is used for higher concentration ranges (ppm), while GFAAS (Graphite Furnace AAS) enables detection down to ppb or lower. FAAS is faster and suited for routine analysis; GFAAS offers higher sensitivity for trace elements but takes longer per measurement.
No. AAS is typically used for metal and semi-metal elements. For non-metals, alternative techniques such as ICP, XRF, or ion chromatography are recommended.
Background absorption from the matrix or flame gases may interfere with the signal. Techniques like deuterium lamp or Zeeman background correction ensure accurate absorbance measurements.
Key factors include sample preparation, contamination, calibration standards, instrument alignment, and matrix effects. Proper digestion, clean labware, and matrix-matched standards improve reliability.
Because AAS relies on element-specific absorption, each metal has a unique wavelength and calibration curve. Even similar concentrations of different metals produce different absorbance responses.


Example of AAS result table (Pb in drinking water)
| Parameter | Value | Unit |
|---|---|---|
| Sample ID | DW-042 | – |
| Element | Pb | – |
| Measured concentration | 0.012 | mg/L |
| Detection limit | 0.002 | mg/L |
| Method used | FAAS | – |
| Instrument | PerkinElmer AAnalyst 400 | – |

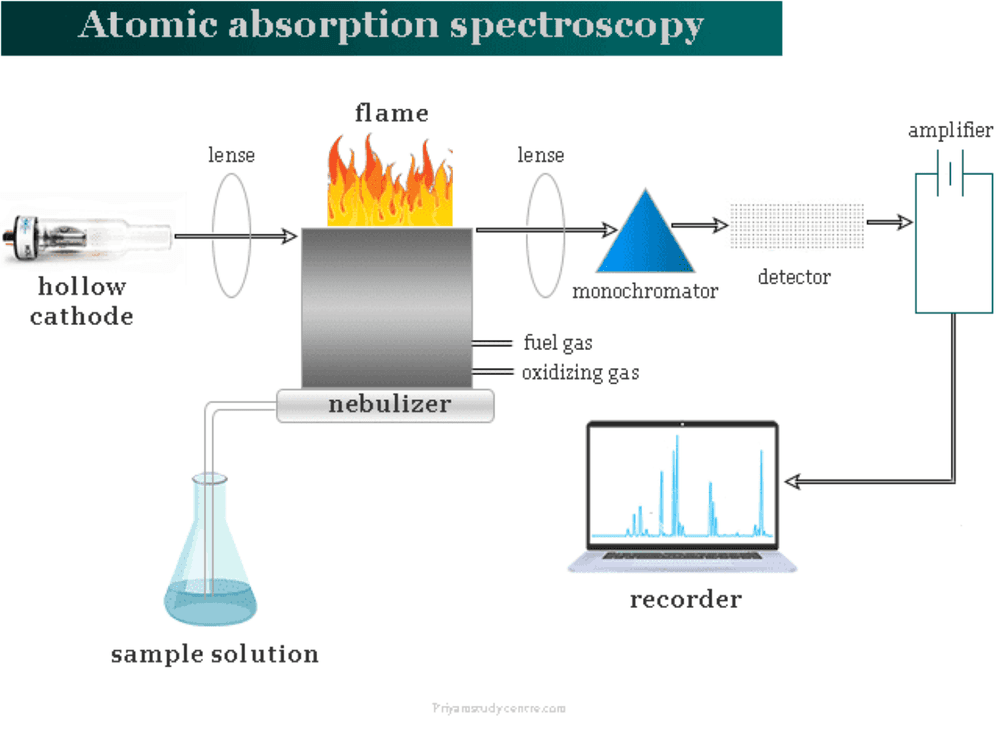
Atomic Absorption Spectroscopy is based on the absorption of light by free atoms in the gaseous state. When a sample containing metal ions is atomized in a flame or graphite furnace, the ground-state atoms absorb light of specific wavelengths emitted by a hollow cathode lamp (HCL) or electrodeless discharge lamp (EDL). The amount of light absorbed is directly proportional to the concentration of the element in the sample.

The AAS analysis process includes the following steps:
AAS (Atomic Absorption Spectroscopy) is a widely used analytical technique for the quantitative determination of metal elements in a variety of sample types. It offers precise and sensitive measurements of elemental concentrations, especially for trace and ultra-trace metals.
The basic principle of AAS involves atomizing the sample—typically in a flame or graphite furnace—and measuring the absorption of light at specific wavelengths by free atoms of the target element. Each element absorbs light at a characteristic wavelength, and the amount of light absorbed is directly proportional to the concentration of that element in the sample.
Typical applications include the determination of heavy metals such as lead (Pb), cadmium (Cd), arsenic (As), mercury (Hg), and chromium (Cr) in water, soil, food, and biological samples. AAS is also commonly used for measuring calcium (Ca), magnesium (Mg), iron (Fe), and zinc (Zn) in pharmaceutical and nutritional products. Industries that widely apply AAS include environmental monitoring, food safety, pharmaceuticals, clinical diagnostics, metallurgy, mining, and chemical manufacturing.