hotline
+41 762172997


Price may vary based on selected options
Delivery time: 1 ~ 2 weeks



Because the powder sample needs to fully cover the entire sample holder; otherwise, the X-rays may hit the holder itself, which would affect the data quality.
Because the size of the sample holder for bulk samples is fixed, and the sample is fixed in place with modeling clay. If the sample is too large, it won’t fit; if it’s too small, it’s difficult to secure.
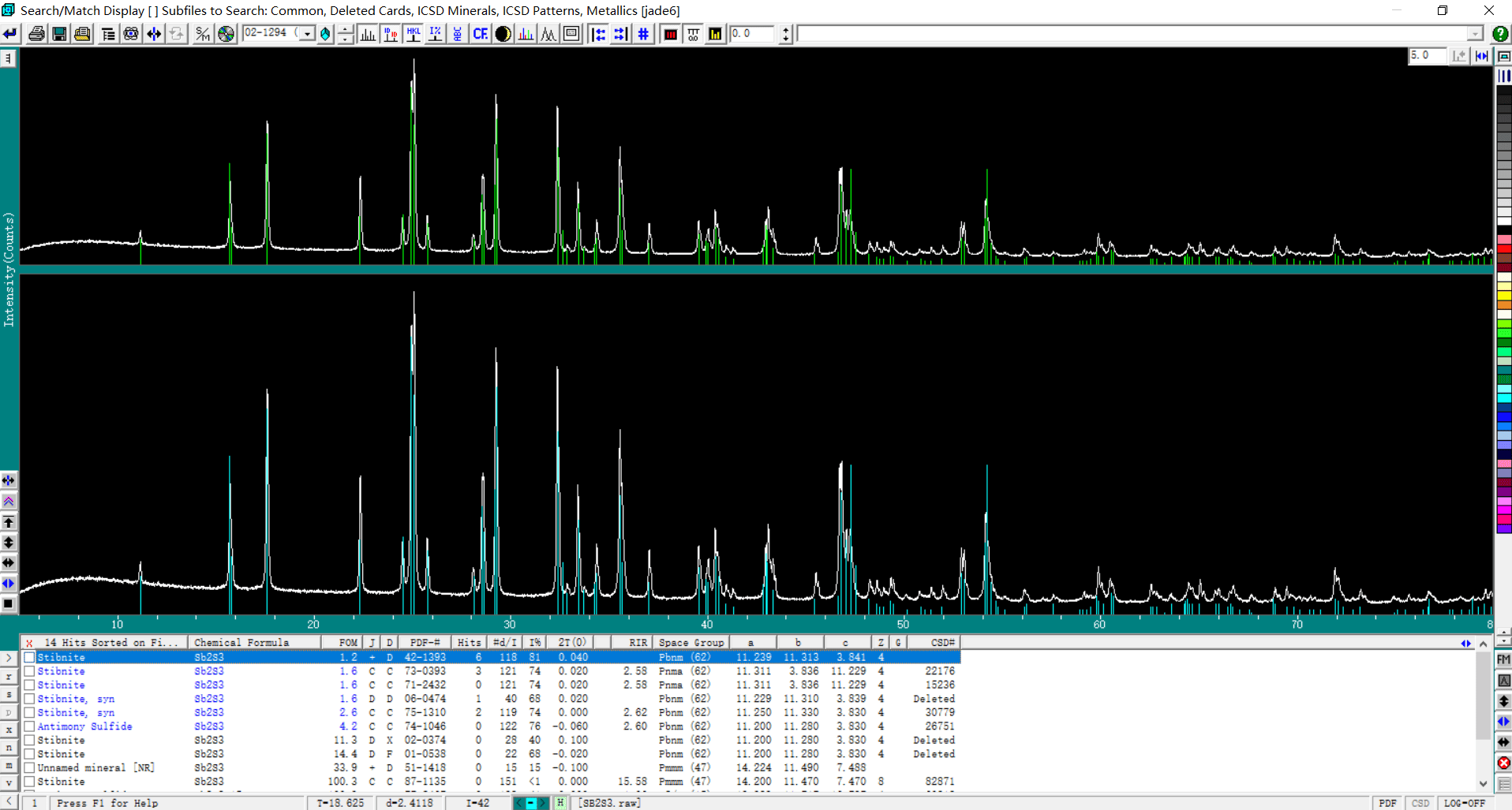
The intensity of the diffraction peaks mainly depends on the crystallinity of the sample itself. It is also related to the amount of sample and the power of the instrument.





The following table presents a comparative overview of several commonly used structural and compositional analysis techniques, including Powder X-ray Diffraction (Powder XRD), Single Crystal X-ray Diffraction (Single Crystal XRD), Electron Diffraction, Raman Spectroscopy, and Infrared Spectroscopy. It summarizes the measurable content, suitable sample types, advantages, limitations, and main application fields of each method.
| Technique | Measurable Content | Suitable Sample Types | Advantages | Limitations | Main Application Fields |
|---|---|---|---|---|---|
| Powder XRD | Crystal structure, phase composition, crystallinity, grain size | Powders, polycrystalline solids | Simple sample preparation, rich information, non-destructive | Insensitive to amorphous materials, quantitative analysis of complex mixtures is difficult | Materials science, inorganic chemistry, minerals, pharmaceuticals |
| Technique | Measurable Content | Suitable Sample Types | Advantages | Limitations | Main Application Fields |
|---|---|---|---|---|---|
| Powder XRD | Crystal structure, phase composition, crystallinity, grain size | Powders, polycrystalline solids | Simple sample preparation, rich information, non-destructive | Insensitive to amorphous materials, quantitative analysis of complex mixtures is difficult | Materials science, inorganic chemistry, minerals, pharmaceuticals |
| Single Crystal XRD | Precise 3D atomic structure | Single crystals | High structural resolution, atomic coordinates obtainable | Requires high-quality single crystals, difficult sample preparation | Molecular structure analysis, pharmaceuticals, proteins, etc. |
| Electron Diffraction | Crystal structure, orientation, defects | Thin films, nanomaterials | Micro-area analysis, high spatial resolution | Requires electron microscope, sample must withstand electron beam | Nanomaterials, semiconductors, catalysts |
| Raman Spectroscopy | Molecular structure, chemical bonds, crystal form | Various solids, liquids | Non-destructive, easy operation, can analyze organic/inorganic | Sensitive to water, fluorescence interference | Materials, chemistry, pharmaceuticals, minerals |
| Infrared Spectroscopy | Molecular structure, functional groups | Various solids, liquids | Easy operation, sensitive to organics | Not sensitive to inorganic crystal structure | Organic chemistry, polymers, pharmaceuticals |
Powder X-ray diffraction (XRD) is a precise analytical technique for determining the crystal structure and phase composition of materials. By analyzing the diffraction patterns produced when X-rays interact with powdered samples, Powder XRD reveals key information such as crystal form, lattice parameters, crystallinity, and grain size. Widely used in fields like materials science, chemistry, geology, and pharmaceuticals, it is essential for phase identification, structural analysis, and quality control of solid materials due to its efficiency, non-destructive nature, and rich data output.